
Boluojia® is a biosimilar to XGEVA®.
(This product is a prescription drug, please take this drug with doctor's prescription and under doctor's instruction)
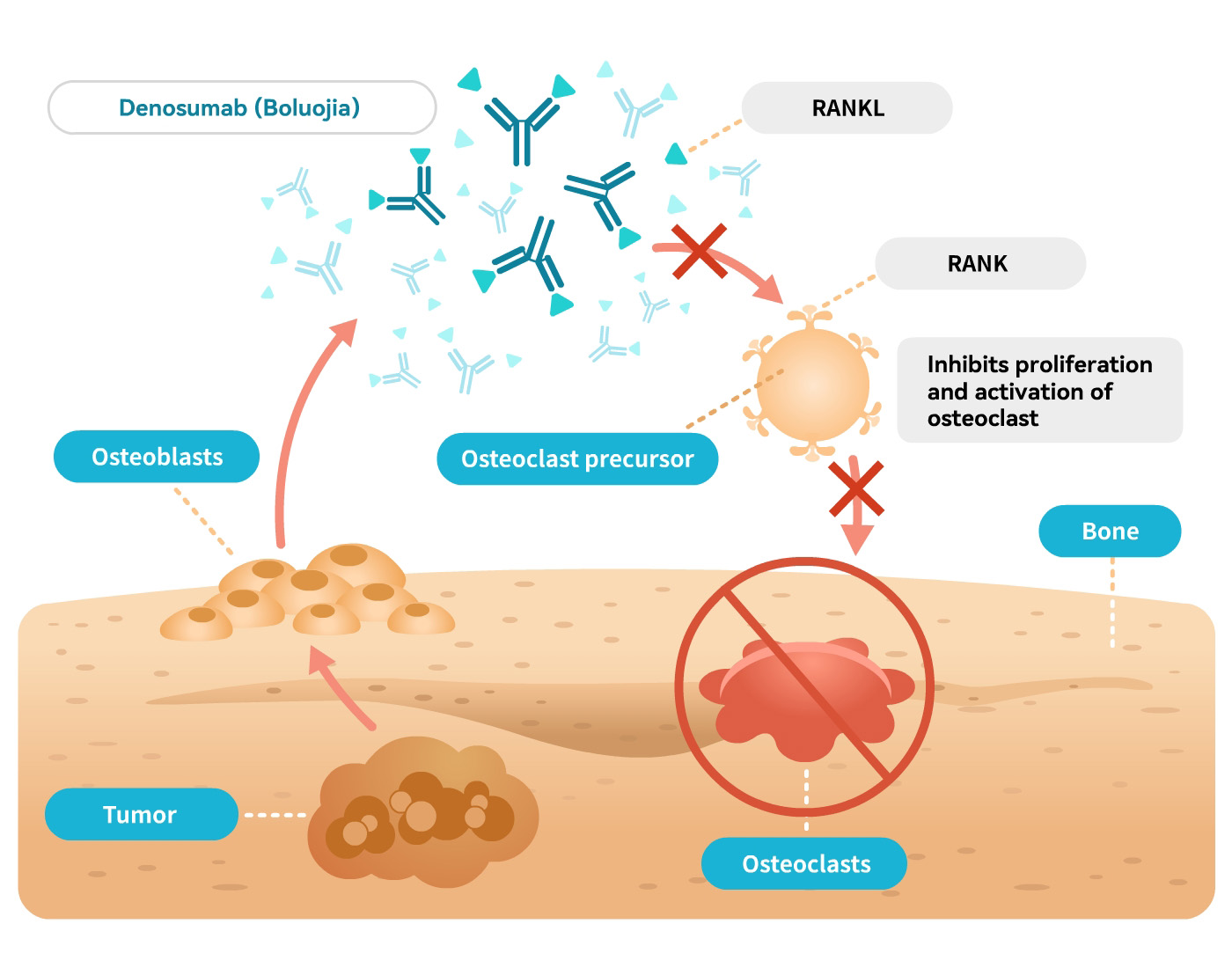
Denosumab can block RANKL to activate the osteoclasts and the receptor RANK on their precursor surface. Blocking the interaction between RANKL and RANK can inhibit the formation, function, and survival of osteoclasts, thereby reducing bone resorption and increasing bone mass and strength in the bone cortex and trabeculae.
References:
1. Zaj,c,kowska R, et al. Int」Mol Sd. 2019, 20(23):6047
2. Shapiro CL,et al. J Clin Oncol. 2019, 37(31):2916-2946
3. 中国临床肿瘤学会(CSCO)乳腺癌诊疗指南2023
4. 中国临床肿瘤学会(CSCO)非小细胞肺癌诊疗指南2023
5. 中国临床肿瘤学会(CSCO)前列腺癌诊疗指南2023
6. 中国临床肿瘤学会(CSCO)恶性血液病诊疗指南2023
7. 中国临床肿瘤学会(CSCO)骨与软组织肿瘤诊疗指南2023
8. NCCN Breast Cancer(Verslon 1.2024)
9. NCCN Non-Small Cell Lung Cancer(Verslon 2.2024)
10. NCCN Prostate Cancer(Verslon 4.2023)
11. NCCN Multiple Myeloma(Verslon 2.2024)
12. Upton A, et al. Eur」Cancer. 2012, 48(16):3082-3092
13. Von Moos R, et al. Support Care Cancer. 2013, 21(12):3497-3507
14. Qian Y, et al. Support Care Cancer. 2017, 25(6):1845-1851
